Need help? We're here to assist you!
Thank You for Enquiry, we will contact you soon!
Close
The Class 10 is an important year in a student’s life and Maharashtra State Board Science 1 is one of the subjects that require dedication, hard work, and practice. It’s a subject where you can score well if you are well-versed with the concepts, remember the important formulas and solving methods, and have done an ample amount of practice. Worry not! Home Revise is here to make your Class 10 journey even easier. It’s essential for students to have the right study material and notes to prepare for their board examinations, and through Home Revise, you can cover all the fundamental topics in the subject and the complete Maharashtra State Board Class 10 Science 1 Book syllabus.

1. Explain the temperature v/s time graph.
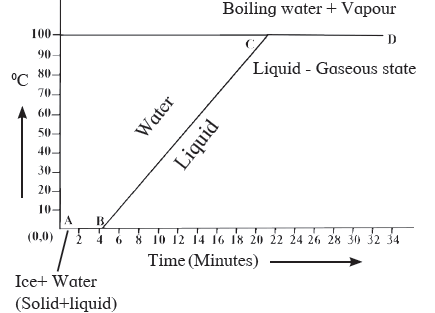
Answer: From the graph above, it is seen that line AB represents conversion of ice into water at a constant temperature. Ice when heated melts at 0o C and then is converted into water maintaining constant temperature of 0o C. Melting point is this constant temperature, at which the ice converts into water. During this transition, the ice also absorbs heat energy. This heat energy is used for weakening the bonds between the atoms or molecules in the ice to transform itself into liquid. This heat energy absorbed by ice, at constant temperature, to convert it into liquid is called the latent heat of fusion. After all the ice is transformed into water, the temperature of water starts to rise. It increases to 100o C. Line BC in the graph represents rise in temperature of water (0o C to 100o C). After this point, even if heat energy is supplied to water, its temperature remains constant. The heat energy is absorbed by water at this temperature to break the bonds between molecules of the liquid and convert the liquid into gaseous state. Thus, during transformation from liquid phase to gas phase, even if heat energy is absorbed by the liquid, its temperature does not change. The constant temperature, when the liquid transforms into a gaseous state is the boiling point of the liquid. The heat energy absorbed at constant temperature during transformation of liquid into gas is called the latent heat of vaporization.
2. What is the role of anomalous behaviour of water in preserving aquatic life in regions of cold climate?
Answer: The anomalous behaviour of water is that from 0o C to 4o C it contracts and beyond 4o C it expands. So, the density of water is at the peak at 4o C. However, when the surrounding temperature goes down, the water in oceans and rivers also cools down and the temperature of whole water reaches 4o C. The water therefore reaches its maximum density at this temperature. Below this temperature (4o C), the water layer on the surface expands caused by anomalous behaviour of the water because of it ’s decrease in density. Hence, this colder layer remains on top and converts into ice. This ice acts as an insulator and does not allow the temperature of the water layer below it to fall below 4o C. This ensures that a liveable temperature is maintained for aquatic life under the oceans and rivers.
3. What is meant by latent heat or latent heat of fusion? How will the state of matter transform if latent heat is given off?
Answer: The amount of heat needed to modify the state of unit mass of the body from solid to liquid or from liquid to gas without resulting in any change in temperature is called latent heat. When the latent heat is given off the body in liquid state, it will convert to solid state and the body in vapour state will transform to liquid state. This means the internal energy of the matter is reduced when latent heat is given off.
4. What is latent heat of fusion or latent heat of vapourization?
Answer: Specific latent heat of fusion is the amount of heat energy absorbed at constant temperature by unit mass of a solid to convert into a liquid phase. Meanwhile, specific latent heat of vaporization is the amount of heat energy absorbed at constant temperature by unit mass of a liquid to transform into a gaseous phase.
5. Explain regelation.
Answer: Regelation is the phenomenon in which the ice transforms to liquid because of applied pressure and then re-converts to ice once the pressure is removed.
6. What is relative humidity?
Answer : Relative humidity is the ratio of actual mass of vapor content in the air for a given volume and temperature to that required to make the air saturated with vapor at that temperature.
% Relative humidity =(actual mass of water vapor content in the air in a given volume / mass of vapor needed to make the air saturated in that volume) x 100
The relative humidity at the dew point is 100%. If the relative humidity is more than
60% the air is humid or if the relative humidity is less than 60%, the air is dry.
7. How much heat energy is necessary to raise the temperature of 5 kg of water
from 20o C to 100o C ?
Answer : Suppose, m= 5 kg and c= 1 kcal/ kg o C
Then, change in temperature Δ t = 100-20=80 o C
Energy to be supplied to water = energy gained by water = mass of water × specific heat of water × change in temperature of water
= m × c × Δ t
= 5 × 1 × 80 = 5 × 80
=400 kcal
Therefore, the heat required to raise the temperature of water = 400 kcal
8. Explain the principle of heat exchange.
Answer: When, heat is exchanged between a hot and cold object, the temperature of the cold object goes on increasing with the gain of energy, while the temperature
of the hot object goes on decreasing due to loss of energy. This change in temperature goes on till the temperatures of both the objects reach the same value. During this process, the cold object gains heat energy and the hot object loses heat energy. If the environment is separated from the system of both the objects by keeping it inside a heat resistant box (meaning that the energy exchange takes place between the two objects only), then no energy can flow from inside the box or come into the box. Principle applied here is Heat energy lost by the hot object = Heat energy gained by the cold object. This is called the ‘Principle of heat exchange.’
9. How will you determine whether air is saturated with vapour or not?
Answer: You can identify if the air is saturated or unsaturated based on the amount of water vapours found in the air. In case the quantity of water vapour is more than what the air can hold, then it is saturated. If the amount exceeds this limit, the excess vapor converts into water droplets. If air temperature is low, it will need less vapor to saturate the air. Same way, if the amount of water vapour is less than the limit that the air can contain, then it is unsaturated. It is also possible to determine if the air is saturated or not in terms of relative humidity. That is, if the relative humidity is 100%,then the air is saturated and otherwise it is unsaturated.
10. What are the melting and boiling points of:
(a) Water/ Ice
(b) Ethyl Alcohol
(c) Gold
Answer: The melting and boiling points of each are given below:
| Substance | Melting point | Boiling point |
| Water/ Ice | 0 o C | 100 o C |
| Ethyl Alcohol | -117 o C | 78 o C |
| Gold | 1063o C | 2700 o C |
Students should passionately focus on the textbooks and practise all the questions and answers in order to secure high marks in the exams. They are recommended to go through all the topics thoroughly. The best resource that the students can find to prepare most effectively for the Maharashtra State Board exam is the MSBSHSE Solutions for SSC (Class 10) of various subjects listed here.